Eurocine Vaccines has signed a Material Transfer Agreement regarding the evaluation of Endocine™ together with a vaccine candidate against COVID-19.
Eurocine Vaccines has signed an evaluation agreement, a Material Transfer Agreement, with an innovative, North American, small public company, regarding the evaluation of Endocine™ together with a vaccine candidate against COVID-19. Studies in one or two animal species will be conducted to study both potency and safety.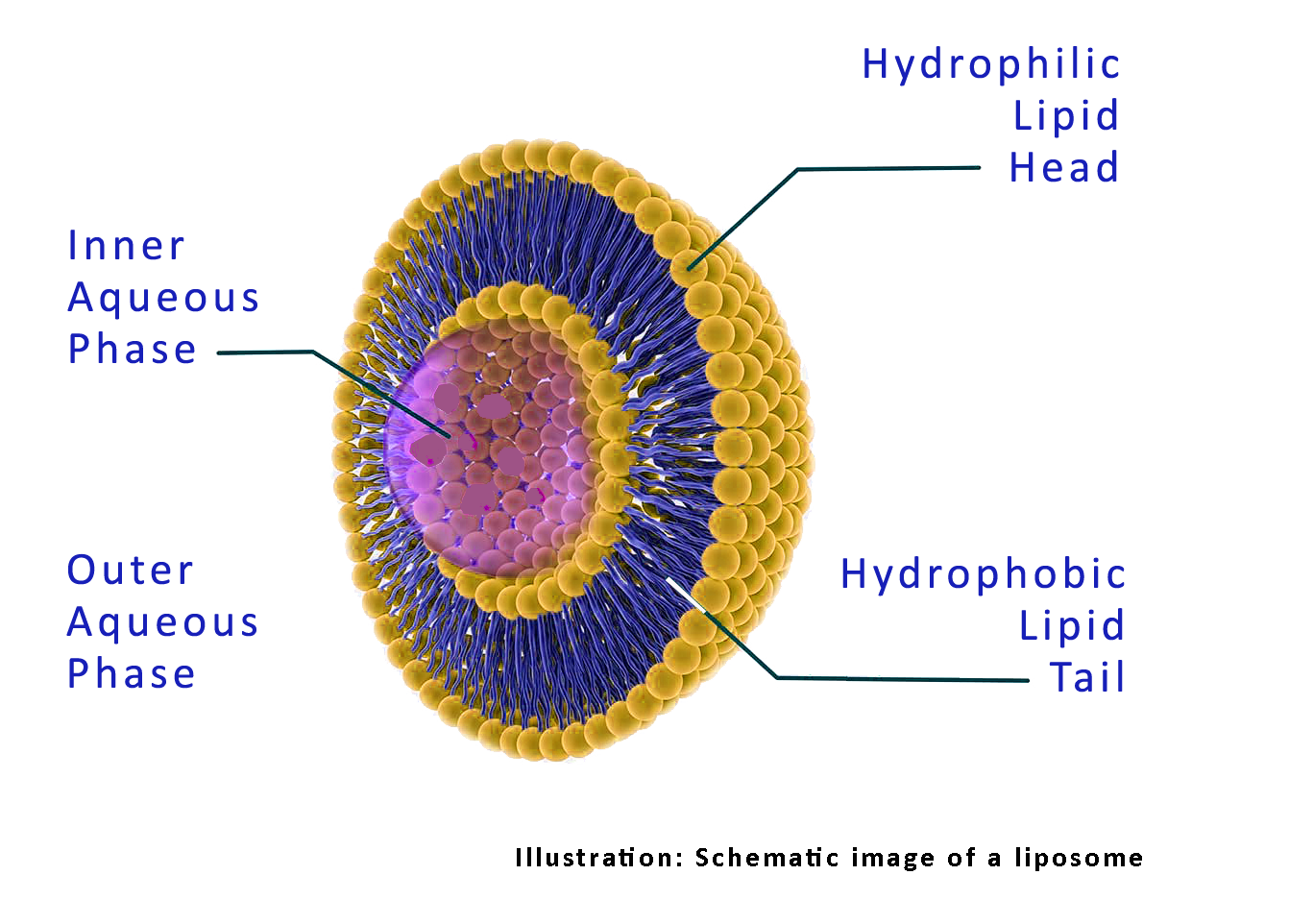
Endocine™ is Eurocine Vaccines’ proprietary adjuvant platform based on natural lipid compounds and formulated as a liposomal dispersion. The manufacture of bulk adjuvant has been established in pilot-scale at GMP conditions and shown excellent safety and tolerability in more than 400 human subjects after nasal administration in five clinical trials.
The platform Endocine™ has been evaluated with several different vaccine antigens, both viral and bacterial, and found compatible with peptides, proteins, polysaccharides, VLPs, as well as split and whole pathogens.
More about Endocine™ can be found at:
https://www.eurocine-vaccines.com/the-portfolio/
If positive data are obtained using Endocine™, the technology may be applied using other vaccine candidates in the collaboration.
